Contributing Writer
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Metallurgy Matters: Phases, structures, and the influences of temperature
- By Bob Capudean
- June 26, 2003
- Article
- Metals/Materials
But phase changes can take place in many metals while still in the solid state. These phase changes are directly related to temperature and take place in the metal's crystalline structure. And while temperature is what controls these transformations, stress, cooling rate, and alloy or chemical composition can all influence the temperature at which the changes take place.
A Closer Look
Remember there are three basic crystalline structures favored by metals: body-centered cubic (BCC), face-centered cubic (FCC), and hexagonal close packed (HCP).
Pure iron is one metal that changes from one of these crystalline structures to another while remaining solid. It's BCC at temperatures up to 1,670 degrees F. But from 1,670 to 2,535 degrees F, it's FCC. Then from 2,535 to the melting temperature of 2,795 degrees F, it goes back to BCC. As these changes take place, the only thing you'll see with the naked eye is color change as the iron gets hotter, until it melts.
This process is called allotropic transformations, and other metals that can go through crystalline structure phase changes while in the solid state include titanium, zirconium, and cobalt.
Of course, as stated earlier, melting and solidifying are also phase changes, they're just not allotropic transformations. And keep in mind that while a pure metal changes from solid to liquid and vice versa at a single temperature, alloys usually change over a range of temperatures. The exception? Eutectic compositions of certain alloys, which solidify at a constant temperature.
Phase Diagrams
Phase diagrams, also known as constitution diagrams or equilibrium diagrams, graphically represent the influences of alloy composition and temperature on phase changes and solidification.
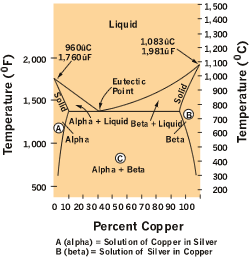 |
| Figure 1 |
In other words, for a given alloy, the phase diagram can show the phases and percentage of each phase present at a specific temperature and alloy composition. It can also show how the phases are affected by changes in the alloy composition, temperature, or both. The problem with phase diagrams is they become complicated with more than a base metal and one alloy.
Figure 1shows a typical silver-copper phase diagram and it tells you a number of things. First, at all temperatures above the liquid line, any combination of silver and copper is liquid. It also identifies where the solid of any combination of silver and copper exits as one or two phases.
A is the silver-rich phase called alpha, B is the copper-rich phase called beta, and both are FCC with different crystal sizes and chemical makeups. The area marked C is where the solid exists as both phases, containing both alpha and beta grains. The two areas between the solid and liquid line indicate where the liquid is in equilibrium with either the alpha or beta phase.
This diagram also identifies the eutectic point, where the compound solidifies at a constant temperature. The interesting thing about eutectic compounds is that alpha and beta phases freeze together alternately. The result is grains of both alpha and beta combined in layers in the microstructure, with an appearance that's obvious under the microscope.
Figure 2shows a typical iron-carbon diagram. Notice that the iron-carbon diagram stops at 5 percent carbon. Why? Because when you get above 5 percent, these alloys are cast irons, not steel alloys, and cast irons can't be hardened. But steel can, and the fact is the iron-carbon diagram is a useful heat-treatment tool.
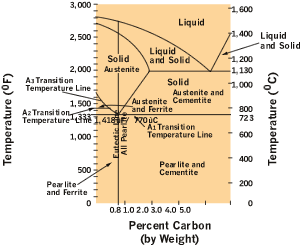 |
The second thing you notice is the number of different microstructures identified at various temperatures and carbon contents. These are the crystalline structures that make up steel at different temperatures.
Ferrite. A solid solution, it's stable at room temperature and capable of containing up to only 0.008 percent carbon at 70 degrees F. Magnetic ferrite is sometimes called alpha iron, not to be confused with the silver-rich alpha phase in the silver-copper phase diagram.
Cementite. This iron-carbon crystalline compound is also called iron carbide. Cementite contains 6.67 to 6.69 percent carbon and can combine with ferrite to form pearlite.
Austenite. Also known as gamma iron, austenite is the FCC form of steel and is capable of dissolving almost 2.0 percent carbon. While austenite is never stable in carbon steel at less than 727 degrees F, additional alloys can make it stable at room temperature. Nonmagnetic and easily work-hardened, austenite is both strong and ductile.
Pearlite. When thin, alternating layers of cementite and ferrite combine, you have pearlite, and it's what austenite transforms to when it's cooled slowly. Pearlite is always 0.77 percent carbon, and it usually makes steel more ductile.
Bainite. Hard with low ductility, bainite is a combination of fine carbon needles in a ferrite matrix. It results when austenite is cooled at a rate lower than what's needed to form martensite.
Martensite. If you take a piece of red-hot steel and quench it in ice water, what you end up with is usually a lot of martensite. Here's why: Martensite results when austenite is quickly cooled to the temperature at which it forms a body-centered tetragonal crystalline structure. If the carbon can't precipitate out of this shear type of structure, which is true for most common steels, it becomes trapped in the body-centered tetragonal lattice—martensite.
This quenched structure is hard, brittle, and basically useless for most commercial steel applications. Tempering will gain back some ductility without costing too much in strength, making it valuable for various tool and die applications. In fact, martensite is what makes it possible to harden steel.
That should give you an idea of how a couple of critical graphic tools are used by metallurgists to predict how steels will react to heating and cooling at various rates. Next time, we'll pick up with hardening and carbon content, steel classifications, and then move to welding metallurgy.
About the Author

Bob Capudean
Back Alley Customs
About the Publication
subscribe now

The Welder, formerly known as Practical Welding Today, is a showcase of the real people who make the products we use and work with every day. This magazine has served the welding community in North America well for more than 20 years.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
Sheffield Forgemasters makes global leap in welding technology

Welding student from Utah to represent the U.S. at WorldSkills 2024

Lincoln Electric announces executive appointments

Lincoln Electric acquires RedViking

Engine-driven welding machines include integrated air compressors

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI



























