- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Our Publications
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
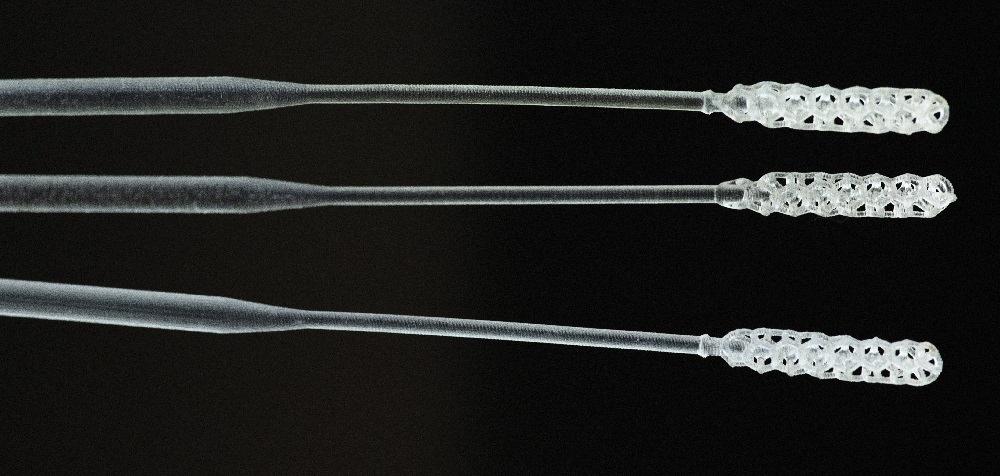
Origin 3D-printed COVID-19 test swabs pass clinical trial
- April 24, 2020
- News Release
- Additive Manufacturing
Origin, the developer of an open platform for additive mass manufacturing, is collaborating with Beth Israel Deaconess Medical Center (BIDMC) on a method for manufacturing COVID-19 test swabs.
Origin recently shifted its resources from being a 3D printer manufacturer to become a medical device manufacturer and has started production to address the shortage of COVID-19 test kits.
BIDMC, an academic medical center affiliated with Harvard Medical School, selected Origin’s 3D-printed test swab to be part of a clinical trial to evaluate it for efficacy and safety. Origin’s 3D-printed swab passed an initial clinical evaluation for human factors, materials testing, and PCR compatibility.
Origin designed the swabs using the nTop Platform to rapidly iterate many different versions for BIDMC to evaluate. The company worked closely with its Open Material Network to identify the proper medical-grade material and to quickly refine optimal processing conditions.
It also is part of a new industry consortium called PrintedSwabs.org, which is bringing together the efforts of the 3D printing industry with academia and medical enterprises to supply millions of 3D-printed COVID-19 test swabs.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI