- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Electropolishing applications and techniques
Process complements mechanical finishing methods
- By Johnson H. Cutchin Sr.
- October 27, 2015
- Article
- Finishing
The early use of electropolishing, practiced commercially since the 1930s, primarily concerned adding cosmetic appeal to consumer goods such as cookware and fountain pens. In recent years, the emphasis has shifted to engineering applications, especially in the food, medical, pharmaceutical, and semiconductor industries. The process, the electrochemical dissolution of a metal surface, is used to improve the metal component’s smoothness, reflectivity, cleanliness, and passivity, or some combination of these surface characteristics. When applied to the ID of pipe system components used in high-purity and ultrahigh-purity applications, electropolishing helps to achieve and maintain the necessary cleanness.
While conventional mechanical finishing processes are macro metal removal processes, electropolishing is a micro process. As such, electropolishing isn’t a competitor with processes such as grinding, sanding, blasting, polishing, and buffing, but rather a complement.
Nearly all metals and alloys can be electropolished, but in practice stainless steel accounts for the greatest portion of commercial electropolishing. The inherent strength and corrosion resistance of stainless steel make it the material of choice for process equipment and many consumer products.
How Electropolishing Works
The part to be electropolished is connected to the anodic (+) side of a direct-current power supply, and the cathodic (-) side is connected to an inert metal, typically lead or stainless steel. Both the part and the cathode are immersed in an acidic solution, and as the current flows through this circuit, metal is dissolved from the metal component. Almost immediately large quantities of oxygen are liberated at the surface of the part, forming a dense gaseous layer. Because of the tendency of electrical current to flow from points and projections, these areas are dissolved preferentially, resulting in a smoothing of the surface. The viscous boundary layer that results from the gassing contributes to the preferential dissolution of peaks and projections. The process is optimized by controlling the solution chemistry, temperature, current density, and time.
Electropolishing generally is considered to be a tank process. Some parts, such as large pipes or vessels, can themselves become the tank for internal electropolishing. Very large, unusually shaped parts that don’t fit into a tank or cannot become a tank require wand electropolishing. In these cases, the part itself is the anode. The electropolishing solution and cathode are brought to the part using a device colloquially called an electrified paint brush.
In most cases, the size and configuration of the parts to be electropolished is limited mainly by the imagination of the electropolishing technician.
- Part size varies from small (hollow needles) to large (15,000-gallon vessels).
- Some parts can be introduced directly into the electropolishing system, while others may require pretreatment to remove grease, soils, or scale. After processing, the parts must be thoroughly rinsed to remove the electropolishing solution.
- Simply shaped parts may be electropolished by using general-purpose cathodes, located several inches or more away from the part. Complex shapes may require the use of elaborate and complex fixtures and purpose-built conforming cathodes to obtain uniform electropolishing over the entire part or to electropolish selected areas.
- Electropolishing solutions are highly acidic and corrosive, requiring corrosion-resistant equipment and close attention to worker safety in personal protective equipment and work practices to prevent contact with the solution.
- Another safety consideration concerns gas buildup. The process generates oxygen and hydrogen; agitating the solution prevents gas entrapment or collection. Furthermore, they must be allowed to escape to the atmosphere and confined in a space. Should they become trapped and not allowed to escape, they would displace the electropolishing solution and could explode if ignited by a spark.
- Electropolishing of large parts can require current up to several thousand amperes, necessitating the use of large rectifiers and heavy cables or busbars. In addition, it can be necessary to use a cooling system to maintain the temperature of the solution and the electrical connectors.
- Metal removal on a plane surface is generally less than one thousandth of an inch, but it’s not uncommon to remove up to 10 times this amount on sharp edges. For this reason, electropolishing often is used for deburring. In general, curved forms and complex shapes electropolish better than flat surfaces.
- Electropolishing times range from one to 20 minutes, although longer times are sometimes necessary.
New or repeat parts are best handled in a properly equipped shop, whereas items that are one-off, very large, or otherwise difficult to transport can be electropolished off-site. Off-site electropolishing isn’t necessarily more hazardous than electropolishing in a tank, but it does require special attention to some factors. The chemicals are highly corrosive and hazardous. Further, the rinse water is considered a hazardous waste, so it’s best if the site has waste treatment capability to handle a rinse that contains trivalent chromium, nickel ions, and dilute acid.
If such facilities are not available at the site, it’s necessary to collect the rinse water. A one-time EPA generator permit must be obtained and arrangements made for transporting this hazardous waste to an approved and licensed facility.
Surface Improvements
Electropolishing can produce mirrorlike reflective surfaces that enhance a product’s appeal and maintain its appearance indefinitely. Using the standard measure, roughness arithmetic average (RA), which is measured in microinches (µin.), electropolishing often reduces the surface roughness by about one-half. That is, a surface with a 100-µin. RA often can be improved to 50-µin. RA, and a 10-µin. RA often improves to 5-µin. RA.
However, this guideline is subject to diminishing returns. As the initial surface becomes finer and finer, the subsequent electropolished surface shows less improvement. In some cases, electropolishing can actually increase the surface roughness by exposing voids or inclusions that previously had been smeared over and obscured by mechanical finishing.
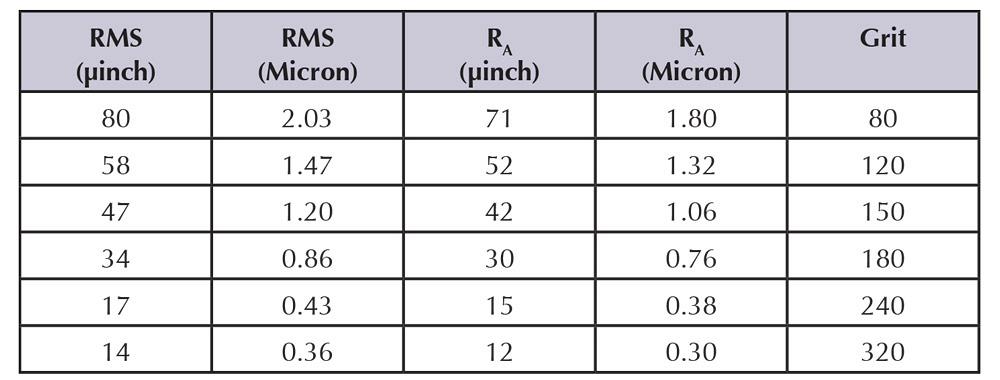
Figure 1
These values are average data from a large number of tests. Because many variables
influence the data, deviations of ±5 percent would be considered within good measurement
parameters.
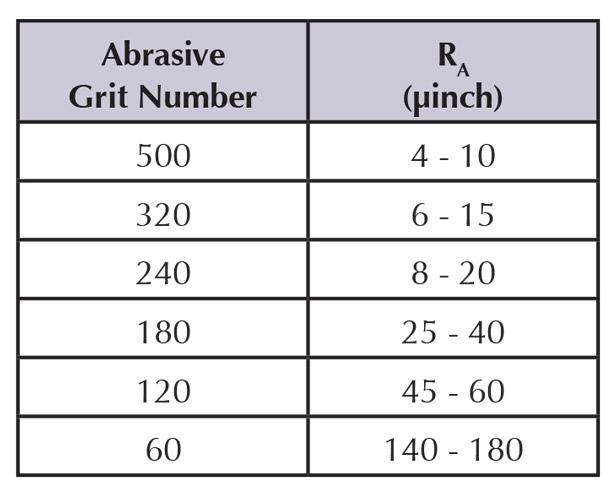
The smoothness achieved by electropolishing corresponds more or less to a grit number of common abrasives (see Figure 1).
The relationship between abrasive grit number and surface roughness, measured in microinches, occasionally is used to specify surface smoothness (see Figure 2).
Surface Characteristics. Electropolishing is known to improve three specific surface characteristics, each of which contributes to the surface’s ability to resist corrosion.
First, electropolishing improves a part’s microcleanliness. When examined by a scanning electron microscope (SEM), even the best mirror-bright buffed finish can show smears, tears, and included abrasives, oxides, and debris (see Figure 3). The same surface after electropolishing may not appear different to the unaided eye, but SEM examination at 1,000x magnification typically reveals a featureless surface free from foreign material and abrasive damage. This microcleaning process improves the corrosion resistance of electropolished surfaces.
Second, electropolishing improves the passivity, or corrosion resistance, of stainless steel. As evidence of this, the latest version of ASTM A967 recognizes electropolishing as an accepted method of passivation. While the phenomenon of passivity may not be fully understood, evidence shows that free iron is removed from a thin surface layer, just a few angstroms deep, resulting in a surface richer in nickel and chromium than the parent material. In addition, the chromium and nickel at the surface are more completely oxidized than occurs naturally.
Finally, electropolishing also removes all or part of the mechanically deformed and stressed layer, thereby providing a third mechanism for improved corrosion resistance. This removal of a highly stressed surface layer can also improve fatigue resistance.
In addition, electropolished surfaces are easier to clean, which is particularly advantageous in systems or equipment used for food and beverage, pharmaceutical, and medical applications.
Surface Evaluation. While the reflectivity, sparkle, and shine that electropolishing produces are obvious to the eye, some of the more subtle effects are apparent only through more sophisticated examination, such as surface chemical analysis, profilometry, and in-service performance trials.
Methods for evaluating electropolishing include:
- Scanning electron microscopy (SEM). Ideal for examining the surface for smoothness and to detect features that may allow particles to accumulate, SEM provides tremendous levels of magnification. It also makes more than one image. It is similar to examining the surface of an orange with several cameras and producing a single image of a portion of the orange’s surface.
- Auger electron spectroscopy (AES). A technique that analyzes the atomic composition of an alloy, this tool is powerful but does have a couple of limitations. First, it determines the identities of the elements present on the surface only. Second, it can’t differentiate metal chromium from trivalent or hexavalent chromium.
- Electron spectroscopy for chemical analysis (ESCA). A tool for determining the composition of the oxide, ESCA can differentiate various forms of the elements present, such as metal, trivalent, and hexavalent chromium. Measurements can also be made at various depths from the surface. This is similar to determining the various constituents in one layer of an onion. The data collected can be used to express chromium-to-iron ratios, the thickness of the oxide layer, and degree of oxide formation in the layer. This method provides data that can be presented in the form of charts or graphs.
In combination with other techniques, it is possible to use AES to examine various depths or layers. This is similar to determining the elements in one particular layer of an onion. This method provides data that can be presented in the form of charts, graphs, or trend lines.
Preparation for Electropolishing
Some parts can be electropolished with no mechanical preparation. For example, some mill finishes produce excellent electropolish results without mechanical work beforehand. The general-purpose cold-rolled 2B finish comes out bright, smooth, and clean, although any nicks or scratches from handling and fabrication will need mechanical finishing beforehand to produce a uniform electropolished finish. The 2B finish is actually preferable to mill finishes No. 3 or No. 4, which are produced by relative coarse abrasives, 50 to 150 grit, which will leave visible scratches.
Hot-rolled, acid-pickled finish No. 1 electropolishes shiny and white, but will reveal many of the depressions and undulations that result from the hot-rolling and descaling process. Producing a mirror finish on this material requires many passes with progressively finer abrasives before electropolishing.
Sand-, grit-, or glass bead blasting generally produces surfaces too coarse to be smoothed completely by electro-polishing. SEM examination of blasted surfaces after electropolishing shows the surface to be decontaminated and smoothed, but the major disturbances are still present.
Similarly, surfaces polished with coarse abrasives may never have all of the scratches removed. For the most part, electropolishing completely smooths abrasive scratches from 220 grit and finer, but scratches from anything coarser than 220 grit remain visible. Note that preparation with an abrasive finer than 320 grit doesn’t always result in a finer finish after electropolishing.
Electropolishing can be an excellent tool for burr removal. In some cases, it’s the only economical method. A burr inside a very small drilled, pierced, or tapped hole is an example. Electropolishing can remove such a burr and, because the process provides a preferential removal of projections, it does so without altering the part’s dimensions.
Some burrs do require mechanical removal. A pierced hole may have a burr perpendicular to the surface, one that is too large to be removed by electropolishing. A mechanical operation such as belt lapping can be used to remove the majority of the burr, leaving a small, sharp burr in the hole. The remaining burr can then be removed by electro-polishing. Burrs most amenable to removal by electropolishing are those that are small, sharp, and difficult or impossible to remove mechanically.
Electropolishing is also a valuable tool for producing fine radii, for example where two ground surfaces meet. It can remove any fine burr and leave a small but definite radius. Electropolishing can be considered a precision machining method, especially suitable when it is necessary to remove a very thin layer of metal to achieve a precise dimension with a fine surface finish.
Welding before electropolishing can be a particular problem, which stems from the welding process and the welder’s skill. On one hand, a weldment might have voids and inclusions that when removed reveal more of the same. On the other hand, top-quality welds often can be electropolished satisfactorily with no prefinishing. Passivation before electropolishing may be necessary to produce the best results (see Figure 4).

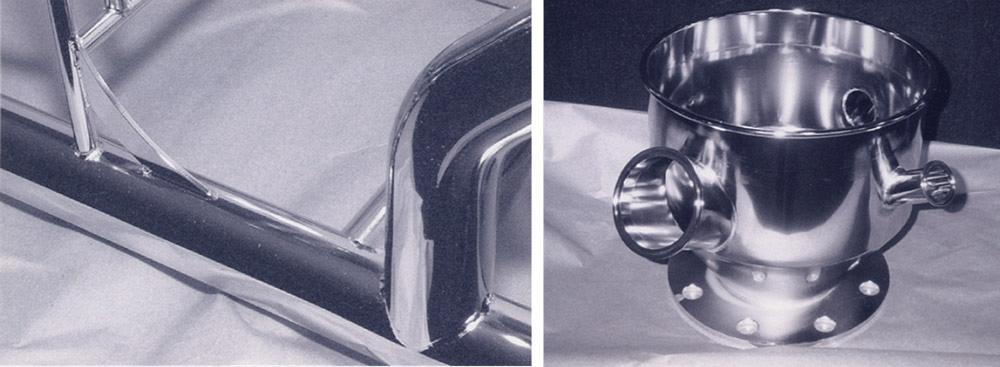
Figure 3
A mechanically finished surface often looks flawless to the unaided eye, but magnification
tells a different story (left). Electropolishing can eliminate nearly all of these microdefects
(right).
Furthermore, phase changes, alloy segregation, and carbide precipitation may become more noticeable after electropolishing, particularly when these changes are caused by welding and are in limited areas. In some cases, these problems can be minimized in the same way, by passivation before electropolishing.
Packaging
If the packaging materials and processes for sending components to the electro-polisher aren’t chosen and used carefully, they can create problems during the subsequent processing. In addition, insufficient packaging can permit damage during transit that may not be overcome during processing.
Bubble wrap is a suitable packaging material, but it shouldn’t come into direct contact with the metal component. Chemicals in the wrap leave a pattern that becomes evident during processing. A best practice following electropolishing is to wrap the parts in acid-free, sulfur-free paper. If necessary, the parts then can be overwrapped with bubble wrap.
It is common practice among metal finishers to return parts in the same packaging they arrived in. This should be considered when preparing parts for shipment to the metal finisher.
Protective coatings must be completely removed before electropolishing, especially from areas subject to heating such as during welding. The adhesive film must be completely removed, usually by wiping with a solvent such as acetone, before mechanical finishing or electropolishing.
During subsequent handling or installation, care should be taken to avoid contact with carbon steel or nonpassivated stainless steel. Such contact can result in the transfer of free iron, which degrades the part’s passivity.
Recent Trends and Applications
A great deal of interest has developed over the last several years in producing extremely clean and corrosion-resistant components for use with ultrapure gases and liquids. Electropolishing has proven to be an effective method in this field. Piping, valves, vessels, pumps, and other components used in handling ultrapure gases and liquids often are mechanically finished and then electropolished to surface roughness values that are extremely low. The final evaluation of the electropolishing consists of two components: the surface appearance and the surface chemistry. The first evaluation is intended to determine the efficiency of the polishing action itself; the second is to ensure that the resulting surface is properly passivated and protected as much as possible.
Another innovative application that has potential is electropolishing as preparation for physical vapor deposition (PVD) coatings. Some evidence indicates that PVD coatings may be applied over electropolished stainless steel surfaces with greater success than over nickel-chromium plated surfaces. One of the reasons for this successful application is that electropolished surfaces are virtually featureless and provide an extremely clean surface for the PVD coating. Most of these applications also include mechanical polishing or buffing on at least selected areas followed by electropolishing before PVD coating.
About the Author
Johnson H. Cutchin Sr.
510 Saco-Lowell Road
Easley, SC 29640
864-859-9314
About the Publication
subscribe now

The Tube and Pipe Journal became the first magazine dedicated to serving the metal tube and pipe industry in 1990. Today, it remains the only North American publication devoted to this industry, and it has become the most trusted source of information for tube and pipe professionals.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI