- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Batch hot-dip and inline galvanizing
A tale of two processes
- By Philip G. Rahrig
- April 11, 2002
- Article
- Tube and Pipe Fabrication
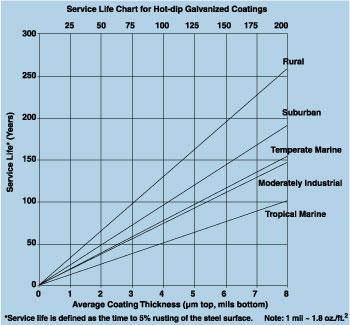 |
| Figure 1 |
The two most common methods of applying zinc metal to steel handrail tubing for corrosion protection are batch hot-dip galvanizing and inline, or continuous, galvanizing.
Batch hot-dip galvanizing involves loading a rack or overhead lifting fixture with 50 or more cut lengths (usually 20 or 40 feet) of tubing, cleaning the steel, and then immersing the entire load into a molten zinc bath that thoroughly coats all exterior and interior surfaces. Once the newly galvanized tubing cools, it is ready for shipment to the job site or transport to a paint contractor for painting.
Inline, or continuous, galvanizing involves feeding the tubing through a bath of molten zinc and then applying a conversion coating to prevent the formation of naturally occurring zinc oxide and hydroxide. A clear, inorganic, polymeric paint topcoat usually is applied over the conversion coating. Only the outside of the tubing goes through this process; the tubing's inside surface receives only a coating of zinc-rich paint.
Fundamentally, zinc metal provides some level of corrosion protection to steel used in myriad applications. However, understanding the metallurgy, bond strength, corrosion mechanisms, and testing of these two methods might lead to better design decisions for particular applications.
Zinc Coating Thickness and Service Life
On standard 0.25-inch-wall tubing, the batch hot-dip galvanizing process applies a minimum of 3.0 mils (1.7 ounces per square foot) of protective zinc. The inline process applies about 0.9 mil (0.5 oz. per sq. ft.). The service life chart for zinc coatings (see Figure 1) shows the approximate service life, depending on environment, for batch-processed and inline zinc coatings. A hot-dip galvanized coating can be expected to last approximately three times longer than an inline-applied coating.
However, the longer service life may come with a higher price tag. Hot-dip galvanizing requires more zinc and more labor, so it may be more expensive than inline galvanizing.
Applying a barrier protection system over a galvanized coating (applied either by batch or inline) results in a service life 1.5 to 2.5 times the sum of the service life of the two systems. While applying a barrier coating to the inline-galvanized handrail enhances its service life, it does not match that of the unpainted, batch-galvanized handrail. And, because the interior of inline-galvanized handrail does not have a metallurgically bonded zinc coating, it is difficult, at best, to compare service life expectancies precisely.
Bond Strength
Batch hot-dip galvanizing generates a metallurgical reaction between the molten zinc and the iron in the steel tubing. This reaction causes the formation of three zinc-iron alloy layers that are metallurgically bonded to the base metal and normally are topped by an impact-resistant pure zinc outer layer.
The metallurgical bond between the zinc-iron alloy layers and the steel is measured at approximately 3,600 pounds per sq. in. (PSI). This bond strength is especially important for the interior surface of batch-galvanized tubing, where the coating resists corrosion caused by trapped water and moisture.
In comparison, the zinc-rich paint applied to the inside of inline-galvanized tubing has a mechanical bond in the range of 300 to 500 PSI. Because of this lesser bond strength, it is possible for trapped moisture to make its way between the zinc-rich paint and the steel tubing, causing rust formation and eventual flaking and failure of the interior paint system, which cannot be repaired or reapplied.
Corrosion Protection
Batch hot-dip galvanizing metallurgically bonds zinc to all surfaces of the tubing. In fact, batch-galvanized tube and pipe often are used in fabrications with hot-dip-galvanized vessels and tanks to store a variety of liquids or any trapped moisture. The zinc-rich paint applied to the inside of tubing galvanized inline provides some corrosion protection but performs less effectively when exposed to liquids. In such cases, cracks, damaged areas, and the porosity of the zinc-rich paint permit moisture to contact the base steel of the tubing, allowing corrosion to begin where it is not apparent and is not easily remedied.
Inspection and Testing
Salt-spray or salt-fog tests often are used to compare the corrosion protection various coatings provide. These tests have no relationship to actual service life and therefore can be used only for a relative comparison in this service condition.
After being put into service, galvanized coatings develop a protective zinc-carbonate patina after several wet and dry cycles. This naturally occurring patina increases the coating's long-lasting corrosion protection. It is widely understood that laboratory salt tests are not reflective of real-world situations. So it is unrealistic and ill-advised to use salt-spray tests to develop a realistic comparison between batch hot-dip-galvanized tubing without an additional barrier protection system and inline-galvanized tubing that is additionally conversion-coated and top-coated with inorganic polymeric paint.
Durability
The more than 3 mils of zinc in the zinc-iron alloy layers applied during batch hot-dip galvanizing are actually harder (250 diamond-pyramid number [DPN]) than the base steel (159 DPN). It is extremely difficult to damage the thick zinc coating to the extent that corrosion protection would be affected.
In comparison, the inorganic polymeric paint covering the thin layer of zinc applied inline can be damaged as easily as any other paint and is particularly susceptible to deterioration caused by the sun's ultraviolet (UV) rays. Any damage to the paint covering exposes the thin layer of zinc. This type of protection system most likely will last only as long as the zinc thickness will allow, or about a third as long as the batch hot-dip-galvanized tubing. Additionally, surface contaminants and normal in-use wear and tear are particularly threatening to topcoat paints.
Touchup and Repair
Batch hot-dip galvanizing is done after the tubing is cut to length and protects all surface areas. Touchup is rarely required after transport and field construction because the thick zinc coating is hard and bound tightly to the steel surface.
Inline-galvanized tubing is cut to length after galvanizing. The unprotected ends are susceptible to corrosion if not touched up with zinc solder, metallizing spray, or zinc-rich paint and will be potential sites of structural failure.
Making a Choice
An architect's or engineer's decision to use batch hot-dip-galvanized tubing or inline, continuously galvanized tubing depends on the design life desired for the handrail and funds available for future maintenance.
While hot-dip galvanizing has advantages in service life, bond strength, corrosion protection, and durability, inline galvanizing may offer an initially more affordable coating alternative for applications in milder environments and projects that have lower bond strength requirements.
About the Author
Philip G. Rahrig
6881 South Holly Circle, Suite 108
Englewood, CO 80112
720-554-0900
About the Publication
subscribe now

The Tube and Pipe Journal became the first magazine dedicated to serving the metal tube and pipe industry in 1990. Today, it remains the only North American publication devoted to this industry, and it has become the most trusted source of information for tube and pipe professionals.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
Team Industries names director of advanced technology and manufacturing

Orbital tube welding webinar to be held April 23

Chain hoist offers 60-ft. remote control range

Push-feeding saw station cuts nonferrous metals

Corrosion-inhibiting coating can be peeled off after use

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI


























