Senior Product Manager, R&D international
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Passivation basics: Will this stainless steel rust?
How to ensure stainless steel is properly passivated
- By Jonathan Douville
- Updated May 18, 2023
- November 11, 2018
- Article
- Testing and Measuring

An automated arm electrochemically cleans to ensure complete passivation occurs along a stainless steel weld joint.
In the world of stainless steel fabrication, stainless steel should mean just that—stainless. Yet it’s not unusual for fabricators to complain about the appearance of rust after commissioning or installing components. These on-site repairs can be costly. For customers, it can mean headaches and expensive delays. Rust is bad for business, which is why passivation is essential. In chemistry and engineering, passivation refers to a material becoming passive, or less affected by the environment.
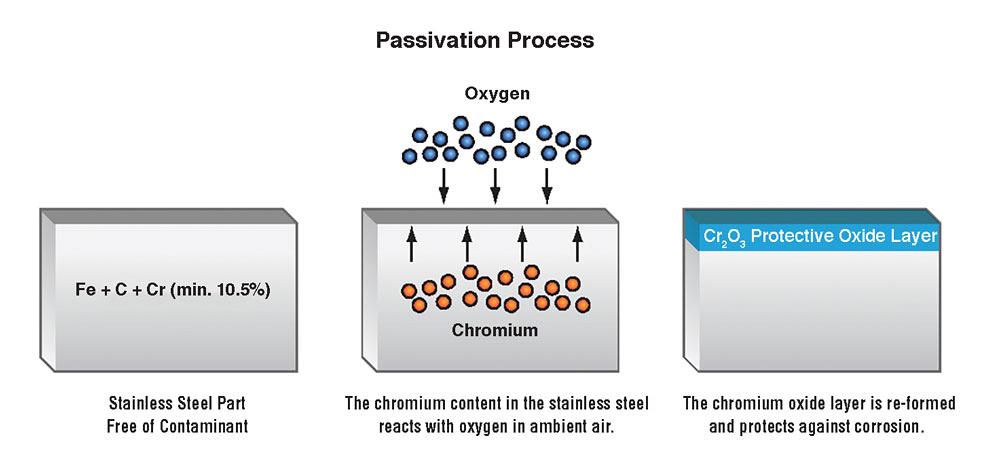
What makes stainless steel stainless? In a word, chrome. Stainless steel is an alloy of iron with a minimum of 10.5 percent chromium. Chromium produces a thin layer of oxide on the surface of the steel—the “passive” layer—that prevents surface corrosion.
Passivation is an essential process in the manufacture and quality assurance of varying grades of stainless steel. It begins immediately after surface contaminants are completely removed. In stainless steel it typically takes 24 to 48 hours to achieve a uniform and stable passive layer, but passivation can occur only in certain conditions. It’s not uncommon for the passive oxide layer of stainless steel to sustain damage through any number of mechanical, industrial, and environmental processes. This is why passivation is the final step in stainless steel parts manufacturing.
How Does Stainless Steel Oxidize?
Stainless differs from carbon steel by the amount of chromium present. Unprotected carbon steel rusts when exposed to air and moisture. This iron oxide film (rust) is “active” and accelerates corrosion by making it easier for more iron oxide to form.
Stainless steel contains enough chromium to undergo passivation by forming an inert film of chromium oxide on its surface. But passivation occurs only if the proportion of chromium is high enough and oxygen is present.
Increasing the amount of chromium increases resistance to corrosion. Stainless steels also contain varying amounts of carbon, silicon, and manganese. Added elements such as nickel and molybdenum can impart other useful properties like enhanced formability and increased corrosion resistance.
Some metals such as gold and titanium are self-passivating. Exposed surface atoms readily react with oxygen in ambient air to form a stable layer of passive metal oxide. Yet if steel tools are used on such metals, trace amounts of free iron (ferric material) can be left on the surface, and the iron will corrode. The same is true for stainless steel.
To passivate, stainless steel surfaces must be free of contaminants like free iron. A clean surface allows the chromium to react with oxygen in ambient air to form an inert, or passive, layer of chromium oxide on the metal’s surface. The chromium oxide microcoating acts as a barrier between the iron-dense alloy and the ambient air.
Cleaning to Enable Passivation
Welding creates a heat-affected zone and heat tint in which the alloy structure of stainless steel is altered. Heat tint is a thickening of the naturally occurring oxide layer on the surface of stainless steel. As heat tint colors are formed on stainless steel, chromium is drawn from below the surface of the metal to form a chromium-rich oxide surface layer.
This leaves the metal just below the surface with a lower chromium level, which can negatively affect corrosion resistance. Heat tint is a serious contaminant that must be removed from the surface, not only for aesthetic reasons but also to allow stainless steel to self-passivate.

A technician electrochemically cleans a circumferential weld joint on a rolled section of stainless steel to ensure complete passivation occurs.
Sandblasting removes heat tint but embeds contaminants in stainless steel. Grinding, although an effective method to remove heat tint, leaves traces of free iron, which cause pitting and corrosion. Eliminating free iron requires a chemical treatment with harsh acids.
Several cleaning methods exist to enable passivation. However, because of safety, budgetary, and environmental constraints, not all are suitable for fabricators. Methods include treating material with acid solutions, including pickling pastes and gels, which clean the metal surface of free-iron contaminants. Note that these pastes have acids that can be hazardous to the environment and the operator.
Another method involves electrochemical cleaning and polishing. This process removes heat tint and other contaminants, including more iron and nickel, leaving surfaces rich in chrome. Electropolishing attacks peaks and rounds valleys on material surfaces and raises the proportion of chromium at the surface. The technique has a major effect on stainless steel appearances, increasing luster and brightness while only changing measured roughness by about 30 percent.
Some weld cleaning and polishing systems use food-grade acids and electricity to remove heat tint and can achieve chemical passivation on stainless steel parts, as defined by ASTM International.
Validating Passivation and Test Methods
The importance of validating the passivation of stainless steel surfaces cannot be overstated. Impossible to detect with the naked eye, passivation indicates that a protective layer of chromium oxide exists on a stainless steel surface. It’s the essential ingredient that guarantees stainless steel will resist corrosion.
So how does a fabricator ensure stainless steel is indeed fully passivated? A variety of tests exist, and ASTM International describes best practices in its standards. Specifically, the ASTM A380 standard describes best practices for cleaning, descaling, and passivation of stainless steel parts, equipment, and systems. The ASTM A967 standard describes tests with acceptance criteria to demonstrate that passivation procedures have been successful.
A variety of tests for passivation follow. Know, however, that not all of these tests are suitable for all grades of stainless steel.
Water Immersion Test. The water immersion test detects anodic surface contamination, including free iron, on stainless steel. The test exposes passivated components to distilled water in intervals of one hour submerged in water and one hour without being submerged, for at least 24 hours.
Although water is easily accessible, access to specialized immersion chambers can require a significant capital investment. The water must be clean, distilled, and free of chemicals, which may require costly plumbing. Inadequate plumbing can falsely indicate trace iron on tested surfaces. Failed components require reworking and further decontamination. And to comply with the ASTM standard, the test cycle must be at least 24 hours.
Salt Spray Test. The salt spray test is an accelerated laboratory test that provides a controlled corrosive environment to determine the corrosion resistance of stainless steels. It exposes components to a salt spray (fog) solution of 5 percent sodium chloride in a test chamber heated to 95 degrees F. The test duration is short, so it’s not ideal for assessing the behavior of a material, especially stainless steel, exposed to corrosive elements in a natural environment.
The test also is of limited use when comparing the corrosion resistance of different stainless steel grades, such as when establishing a ranking or quantifying the differences in corrosion resistance. The test’s corrosive conditions are fixed and cannot be adjusted for the specific corrosion resistance of certain grades.
The test requires specialized lab equipment that includes a humidity chamber, which may be unsuitable for large stainless steel components. Moreover, failed components require reworking and further decontamination.
High-humidity Test. The high-humidity test detects free iron or any other anodic surface contaminants on stainless steel. It is performed in a humidity cabinet capable of maintaining 97 (±3) percent humidity at 100 (±5) degrees F for at least 24 hours.
The test sample is acceptable if there is no evidence of rust stains or other corrosion. To comply with ASTM standards, components must be immersed in acetone or methyl alcohol and then dried in an inert atmosphere or desiccated container.
This test also requires specialized lab equipment and a humidity chamber, which again may be unsuitable for large stainless steel components. Testing cannot be tailored to different stainless steel grades, and the ASTM standard requires at least a 24-hour testing cycle. And as in other tests, failed components require reworking and further decontamination.
Copper Sulfate Test. The copper sulfate test is rarely accepted in the food industry because of its toxic nature. In fact, ASTM bans the use of this test on stainless steel components “to be used in food processing.”
The test, which requires aqueous copper sulfate solutions “no more than two weeks old” is used for specific grades of austenitic, martensitic, ferritic, and precipitation-hardened steels that are at least 16 percent chromium.
Potassium Ferricyanide-nitric Acid Test. This is recommended when detection of very small amounts of free iron is required on austenitic 200 and 300 series stainless steels. As with the copper sulfate test, ASTM forbids use of this test on stainless steel components used in the food processing sector. It is not recommended for ferritic or martensitic steels because of the false positives the test tends to give on these materials. Also, the testing solution must be prepared daily.
The Open Circuit Potential Test for Passivation
Designed within a portable system that can be brought to the work, this test qualifies the stability and thickness of the passive chromium oxide layer of stainless steel. The test measures the conductivity of two points, with current passing through a liquid in a sensor that helps make the measurement as accurate as possible.
A device displays a numeric value that describes the quality of the passive layer of chromium oxide. A positive value indicates that the sample is passivated, while a negative value indicates it isn’t. The higher the value, the thicker and more resistant the passive layer.

A testing device that operates under the principle of open circuit potential verifies that a stainless steel product is completely passivated.
Passivation Matters
Proper passivation and testing provide documentation—be it manual or digital—critical for any fabricator specializing in stainless steel. Proper documentation serves as an important record that shows workpieces were tested for passivation.
Passivation of stainless steels is a key concern for fabricators, welders, and manufacturers who buy, sell, or work with the ubiquitous and essential material. Thanks to passivation testers, companies have tools in their arsenal to accelerate, detect, and measure passivation, as well as reduce costs associated with rework of rejected stainless steel products.
Jonathan Douville is senior product manager, R&D international, at Walter Surface Technologies, 810 Day Hill Road, Windsor, CT 06095, 860-298-1100. Images courtesy of Walter Surface Technologies.
About the Author
Related Companies
subscribe now

The Fabricator is North America's leading magazine for the metal forming and fabricating industry. The magazine delivers the news, technical articles, and case histories that enable fabricators to do their jobs more efficiently. The Fabricator has served the industry since 1970.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
Capturing, recording equipment inspection data for FMEA

Tips for creating sheet metal tubes with perforations

Are two heads better than one in fiber laser cutting?

Supporting the metal fabricating industry through FMA

Hypertherm Associates implements Rapyuta Robotics AMRs in warehouse

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI