Senior Manager, Product Marketing
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
The chemistry behind mild carbon steel GMAW electrodes
Selecting the optimum filler metal to improve welding productivity
- By Kaar Valluvan
- January 25, 2023
- Article
- Consumables

Selecting a mild carbon steel electrode can greatly enhance or detract from labor productivity and total weld costs—which is why it is important for welders to understand the chemistry behind the filler metal. Images: ESAB
Application experts are often asked, “What’s the best MIG wire?” The answer to this question with gas metal arc welding (GMAW) is always, “One that meets the required welding codes, mechanical properties, and welding procedure specifications.”
However, in addition to meeting technical requirements, fabricators need to consider the total welding cost. Labor constitutes an average of 75% to 85% of total welding costs, while filler metals make up about 10%. Selecting the optimum filler metal can greatly enhance or detract from labor productivity and total weld costs.
The following is an overview of how filler metal chemistry influences welding results, focusing on electrodes for welding mild carbon steel (Grade A36), because they are the most widely used electrodes and the ones that most people learn to use first.
Classification and Chemistry
The American Welding Society (AWS) classifies carbon steel electrodes (see Figure 1). These electrodes have a minimum weld metal tensile strength of 70,000 PSI, making them suitable for welding A36 steel to itself or to other grades of carbon steel (you only need to match the A36 because it has the lowest strength).
When formulating carbon steel electrodes, manufacturers control up to 17 different raw elements (see Figure 2). The three primary alloying elements are carbon, manganese, and silicon. AWS specifies the minimum and maximum quantities of these elements, but manufacturers can emphasize performance attributes by controlling chemistry.
Carbon (C) influences the structural and mechanical properties more profoundly than any other element. For ER70 electrodes, the carbon is usually held between 0.05% to 0.12%, which provides weld metal strength without affecting ductility, toughness, and porosity.
Manufacturers add other alloys to control the deoxidation of the weld puddle and to help determine the weld’s mechanical properties. Deoxidation is the combination of an element with oxygen from the weld puddle, resulting in a slag or silica island formation on the weld surface. Removing oxygen from the puddle eliminates it as a cause of weld metal porosity.
Silicon (Si) is the most common deoxidizing element; depending on the intended use, electrodes generally contain 0.45% to 1% of the alloy. In this percentage range, silicon exhibits very good deoxidizing ability. Increasing silicon will increase weld strength with only a small decrease in ductility and toughness. However, weld metal may become crack-sensitive with more than 1% to 1.2% silicon. The alloy also influences puddle fluidity.
Manganese (Mn) is also a common deoxidizer and strengthener. Manganese constitutes 1% to 2% of mild steel electrodes. Increasing manganese levels increases the weld metal strength to a greater degree than silicon. Manganese also will reduce the weld metal’s sensitivity to hot cracking.
Aluminum (Al), titanium (Ti), and zirconium (Zr) are very strong deoxidizers. These elements are sometimes added in very small doses, usually not more than 0.2% combined. In this range, some increased strength is achieved.
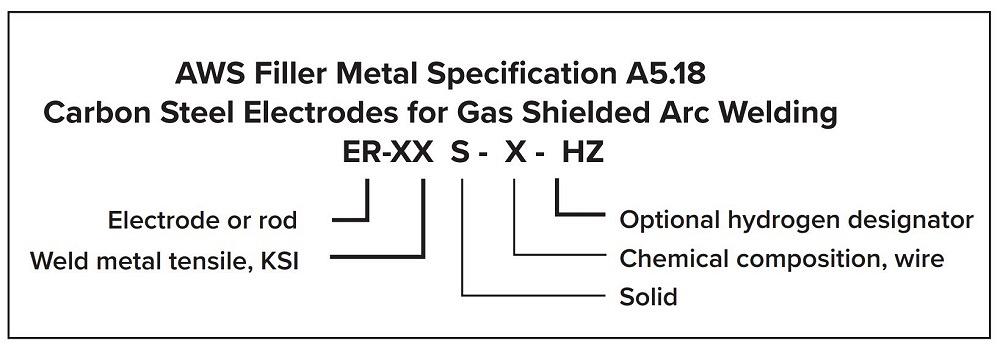
FIGURE 1. AWS classifies carbon steel electrodes based on factors like tensile strength and chemical composition.
Other elements such as nickel (Ni), chromium (Cr), and molybdenum (Mo) are often added to improve mechanical or corrosion resistance properties. In small amounts, they can be used in carbon steel wires to improve the strength and toughness of the deposit.
By the Numbers
GMAW electrodes for mild steel commonly include the letter S (indicating that it’s a solid electrode) and either a numerical designator (2 through 7) or the letter G. The most widely used electrodes are S-3 and S-6.
Here’s a brief guide to what these electrode designations mean and why they are popular, as well as notes on other electrode chemistries to help guide selection.
S-2 “triple-deoxidized” electrodes contain aluminum, titanium, and zirconium (in addition to manganese and silicon) and are designed for welding over rust and mill scale. The less-fluid weld puddle makes it easy to control when used out of position, making it a preferred wire for all-position welding of small-diameter pipe.
S-3 electrodes and others with low silicon levels produce a stiffer puddle and more control of the back bead profile. They tend to produce fewer and smaller silica islands. For painted parts, manufacturers often specify the use of an S-3 electrode.
S-4 electrodes have higher levels of silicon and manganese than S-3 electrodes and are intended for applications requiring higher deoxidizer levels. The ER70S-4 classification does not require Charpy impact conformance testing.
S-6 electrodes have higher levels of silicon, which will create a smoother arc (reducing spatter) and improve puddle fluidity (“wet out”). This promotes a smooth transition at the toes of the weld and flattens the bead crown. S-6 electrodes appeal to users because they make it easier to achieve a good bead and require less cleaning.
S-6 electrodes contain more manganese and silicon, which act as deoxidizers to scavenge impurities. An S-6 electrode performs better than S-3 over small amounts of mill scale, oil, dirt, and rust. The trade-off is that the scavenged impurities manifest as silica islands. Painted parts require cleaning (typically with a needle gun), otherwise the silica island will eventually pop off, create a blemish, and possibly rust.
S-7 electrodes have a higher manganese content, which gives the weldment a higher yield and tensile strength. This electron’s silicon level is somewhere between an S-3 and S-6. They are suitable for use with 100% CO2 or an argon/CO2 shielding gas mixture (100% CO2 reduces the manganese in the weld metal, thereby reducing its strength, which may or may not be an issue depending on the application).
Low-hydrogen electrodes reduce the potential for hydrogen cracking, especially in materials with tougher mechanical properties. An H4R designation indicates less than 4 ml of diffusible hydrogen per 100 g of deposited weld.
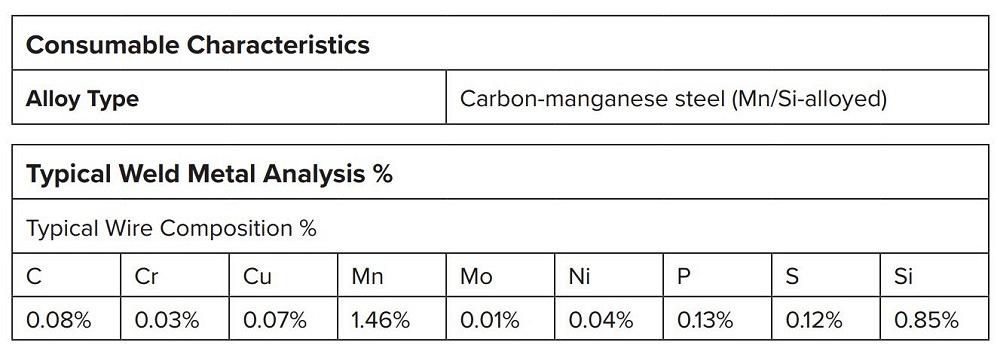
FIGURE 2. Fact sheets list a filler metal’s chemical composition. Shown is the chemical composition of an ER70S-6 electrode used for welding unalloyed steels such as in general structural, pressure vessel, and shipbuilding, and for fine-grained carbon-manganese steels.
G (or GS) electrodes are general classification electrodes that do not have composition, mechanical properties, or testing requirements, but their properties can meet or exceed those of AWS-classified electrodes. They are only intended for single-pass applications, which may include specialty applications like welding galvanized steel.
Electrodes with no classification are often indicative of a specialty application. For example, “easy grind” electrodes are fully deoxidized and designed to weld over the moderate levels of rust and paint found in auto body repair work. The welded metal grinds more easily than most commercial electrodes, making postweld cleaning easier and faster.
Formulation Variations
Manufacturers can emphasize various electrode performance attributes by controlling chemistry, chemistry tolerances, the electrode coating, and the manufacturing process.
As a result, even though electrodes from different manufacturers carry the same designation, their performance can vary widely. Some common examples include:
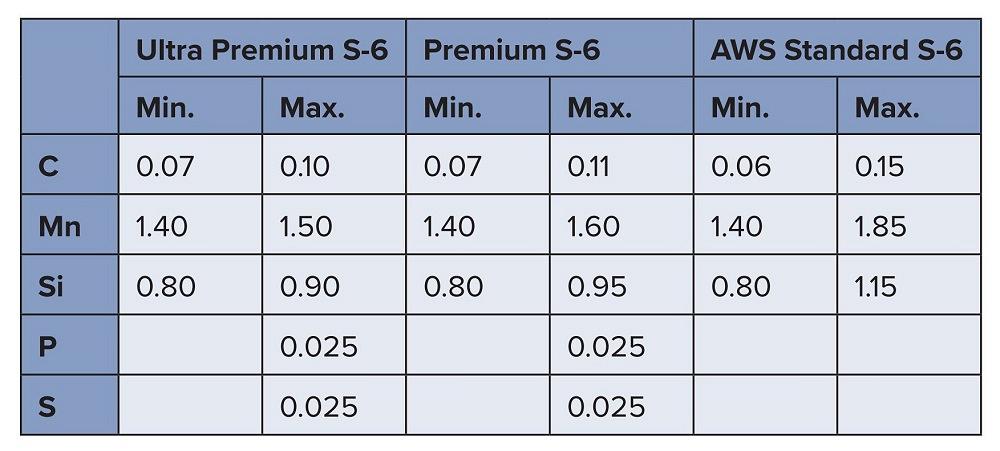
- Consistent chemistry. - High-volume manufacturers and robotic welding applications prioritize consistent and predictable batch-to-batch performance. Figure 3 shows the chemical composition of three S-6 electrodes. The tighter tolerances explain why premium electrodes cost more, but the consistent performance delivers more uptime and lowers total cost of ownership.
- Arc stability. - Spatter and porosity are among the leading causes of grinding, rework, quality issues, and unplanned downtime. Arc instability is a major culprit of these, especially with short-circuit GMAW. Using electrodes with a consistent chemistry promotes arc stability, which in turn will lower total welding costs.
- Galvanized steel. - Wire manufacturers offer chemistries developed for specific application needs. For example, welding galvanized steel generates zinc vapor that can interfere with arc stability, increase spatter, and cause porosity. By controlling micro-alloying elements, they can mitigate zinc vapor issues and improve results.
- Low silica islands. - These wire chemistries enable fabricators to enjoy the puddle fluidity of an S-6 electrode but with a reduced silica island formation—and the islands that do form brush off easily.
Shielding Gas and Chemistry
Shielding gases for GMAW determine the mode of metal transfer and the depth of penetration. In brief, argon and CO2 blends are the most common shielding gases for mild steel electrodes. A high-argon blend (75% to 90% argon, with the balance being CO2 or CO2 and oxygen) achieves good mechanical performance and generates less fume and less spatter, increasing operator appeal.
However, high-argon blends cost more. CO2 is a less expensive gas and provides the broadest penetration profile and the best cleaning action. The trade-off is slightly lower mechanical test results, more welding fumes, a harsher arc, and more spatter.
CO2 and oxygen are useful at times because they promote arc stability and good fusion between the weld puddle and base material. Oxygen is a great deal more oxidizing than CO2. Consequently, oxygen additions to argon are generally less than 10% by volume, whereas 100% CO2 can be used for short-circuit GMAW. When using oxidizing gases, electrodes must contain strong deoxidizing elements to suppress porosity.
Generally, when welding with argon and 1% to 3% oxygen, or with mixtures of argon containing low CO2 content, the weld metal chemical composition will not vary greatly from the analysis of the wire electrode. However, when 100% CO2 and oxygen are used in a shielding gas, a reduction in silicon, manganese, and other deoxidizing elements can be expected, while nickel, chromium, molybdenum, and carbon contents will remain constant. Note that electrodes with very low carbon content (0.04% to 0.06%) will produce a weld metal with a higher carbon content when using 100% CO2.
In many carbon steel applications, the base material has a light layer of mill scale, light rust, or pickling oil that can affect welding performance if not removed before welding. In this case, it is ideal to use an ER70S-6 electrode with a high argon content, such as a 90% argon/10% CO2 blend for spray transfer GMAW or an 80% to 85% argon with CO2 as the balance for short-circuit GMAW. The electrode’s deoxidizers, combined with the 10% to 20% CO2, should provide adequate cleaning action and produce a weld with a good appearance, good penetration, and smooth transitions at the toe of the weld.
Try a Spool or Drum
Filler metal manufacturers continuously update chemistry and packaging as they respond to customer requests to help them address application needs and welding issues. Using a modern formulation can help fabricators address many common causes of unproductive time related to spatter, porosity, silica islands, contact tip wear, and weld quality. In an era where fabricators struggle to find more people, the path to increasing output requires taking every measure possible to keep existing operators welding efficiently, and that includes choosing the optimum filler metal.
About the Author
About the Publication
Related Companies
subscribe now

The Welder, formerly known as Practical Welding Today, is a showcase of the real people who make the products we use and work with every day. This magazine has served the welding community in North America well for more than 20 years.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
Sheffield Forgemasters makes global leap in welding technology

Welding student from Utah to represent the U.S. at WorldSkills 2024

Lincoln Electric announces executive appointments

Lincoln Electric acquires RedViking

Engine-driven welding machines include integrated air compressors

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI