Commercial Director
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Using continuous plasma for cleaning, surface activation of tube, pipe
Glow discharge plasma provides green cleaning and finishing process for welded and seamless products
- By Igor Rogelj and Primoz Eiselt
- January 21, 2015
- Article
- Finishing

Two spools of copper tube show the difference between untreated (left) and plasma-treated (right) surfaces.
Surface treatment processes can be found in virtually every metal shop, and tube and pipe production facilities are no exception. The traditional continuous surface treatment processes are either chemical or mechanical. Chemical processes involve various components such as oils and emulsions to act as lubrications; emulsions to act as cooling agents; acid and alkaline solutions for surface cleaning; and fluxes, primers, and adhesives for surface activation. Mechanical processes such as brushing, sandblasting, and shot peening provide cleaning, surface activation, or surface polishing for surface passivation or for improved visual effect.
The conventional processes have been used successfully for decades, but a plasma-based process provides another option.
Traditional Processes for Continuous Surface Treatment
Traditional surface treatments provide four distinct processes: lubrication and degreasing, deoxidation, surface activation, and surface passivation.
Lubrication and Degreasing. Wet lubricants commonly are used for cold forming or welding of tubes. Lubricants used in tube production are usually oils, greases, and emulsions.
After drawing or rolling, the material has to be degreased to prepare the surface for the next process step. Alkaline solutions such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) commonly are used for degreasing of copper alloys. Alkaline solutions are effective in removing organic substances such as oils and emulsions. For delubrication immediately after a drawing or rolling step, mild alkaline solutions are required. If a lubricated surface undergoes heat treatment without precleaning, the process forms hard-to-remove deposits on the material’s surface. In such cases a more aggressive acid or alkaline treatment is required to clean the surface.
A hot water rinse is usually adequate for removing coolant emulsions if they are removed immediately after application. Other nonconventional surface cleaning and degreasing processes in tube production are ultrasonic cleaning and cotton yarn cleaning.
Deoxidation. Oxide layers on copper alloys are relatively thin (less than 1 micron) compared with oxide layers that form on the surface of steel and aluminum. Copper-alloy oxides traditionally have been removed with various acid solutions such as hydrochloric acid (HCl). High-concentration acids are used for removing thicker, stronger oxide layers that can form on many ferrous metals or hot material exposed to an oxidizing atmosphere. The type of acid used depends on the material as well as the thickness and type of oxide. All acid treatments are followed by rinsing processes and are accompanied with water treatment.
Acid- and alkaline-based processes carry health safety and environmental risks and require proper handling. Acid and alkaline solution storage, handling, and disposal add to the cost of the process operations. Moreover, acids and alkaline substances are difficult, if not impossible, to remove completely from the metal surface. The chemicals that remain after rinsing, even in very small amounts, act as surface corrosion catalysts. Chemically cleaned materials eventually tarnish.
Oxide and scale removal also can be done with aggressive mechanical processes such as brushing, shot peening, and sandblasting, which too can be used for surface cleaning or surface activation prior to coating.
Surface Activation and Surface Passivation. The term surface activation describes the propensity of a surface to adhere to another substance, for example surface coating. Simply stated, an active surface bonds with another substance, whereas a passive surface does not. The terms surface wettability and surface energy are used commonly to describe the level of surface activation.
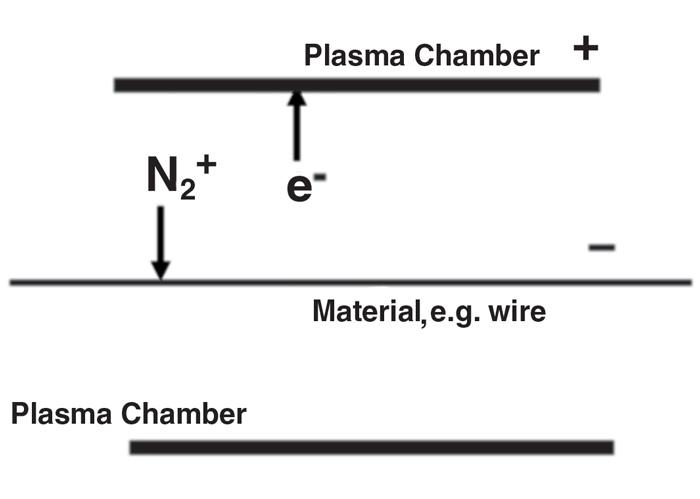
Figure 1
The positively charged outer wall of the heating chamber repels positively charged nitrogen ions, which bombard the surface of the workpiece. The ions with a large amount of energy provide cleaning and annealing; those with less energy improve the surface finish. This process isn’t limited to nitrogen, but can use hydrogen, argon, or helium.
The most common way of increasing surface energy is to increase the surface area by making it rougher. This usually is done by increasing the surface’s microroughness by brushing, sandblasting, or etching, which usually involves a high concentration of ammonia or acid solution.
Deoxidation and degreasing can be described as a form of surface activation. For example, an oxide layer on a copper-alloy surface reduces surface energy by changing its chemical composition and physical properties. Similarly, a lubricated surface prevents a direct connection between the surface and the chosen substance. This in turn prevents good adhesion. For example, to achieve good adhesion in plating, the surface has to be as clean and oxide-free as possible to allow a proper intermetallic bond between the substrate and the plated metal. Acid treatment is therefore an integral part of most conventional plating processes.
In cases such as hot-dip tinning of copper tubes, for example, acid and alkaline pretreatment alone do not suffice. Fluxing is introduced before the plating bath to improve the bond.
Surface passivation is the opposite of surface activation. A passive surface has a low wettability or surface energy and is not prone to react with or stick to other substances. Similarly, a surface with low surface energy has less propensity for corrosion.
Surface passivation is usually achieved with controlled surface oxidation. A thin, homogenous oxide layer on the material surface protects it from reaction with other substances, thereby reducing the likelihood of surface corrosion. Surface passivation can be achieved with polishing, which reduces roughness.
Glow Discharge Plasma Surface Treatment and Its Applications
Glow discharge plasma uses a low-pressure atmosphere for high-temperature heat treatment and surface treatment. The process is based on an electric field that accelerates ions to the surface of the material, bombarding the substrate (see Figure 1). The effect of such bombardment is twofold. The collisions release energy, which is absorbed by the material, providing an efficient heat treatment. Ion bombardment also results in effective surface treatment, the specifics of which depend on the gas used and energy applied. Gases that are used commonly are inert gases such as hydrogen (H2), nitrogen (N2), argon (Ar), and helium (He), either alone or in mixtures. Oxygen (O2) or air also can be used if controlled passivation (surface oxidation) is the aim of plasma treatment. Surface nitriding also can be achieved, for example, if nitrogen plasma is used on an alloy containing nickel.
Glow discharge plasma is used to achieve a variety of effects on the substrate’s surface.
Plasma Degreasing and Surface Cleaning. In the plasma chamber, wet lubricants disintegrate and evaporate, leaving the metallic surface clean and dry. Exhaust gases are removed by vacuum pump filters.
Surface deposits. Plasma treatment removes organic and inorganic surface deposits, leaving the surface free of agents that act as catalysts for surface corrosion. This in turn slows down the process of subsequent surface corrosion.
Oxides. Under the ion bombardment in plasma, the oxide layer breaks up. Plasma effectively removes surface oxides, making it a dry cleaning alternative to the traditional acid-based oxide removal. Oxide removal is effective only at high temperatures, which means that a fair amount of heat treatment must be applied to the material for oxide removal to be effective. Subsequent cooling of the material is also necessary.
Surface activation. Ion bombardment in the plasma chamber increases surface energy to prepare the surface for good coating adhesion. For best effect, the plasma-activated surface should be kept in a protective atmosphere to the point of coating to maximize adhesion.
Surface passivation. Plasma treatment with oxygen or air as the process gas results in surface oxidation. Controlled surface oxidation can be achieved with accurate manipulation of the plasma power input. Good control results in homogenous surface oxide layers with consistent thicknesses.
Plasma cleaning may not always be effective when excessive amounts of dirt are present. In such cases, a conventional cleaning step is necessary before using the plasma process.
Applications. Glow discharge plasma cleaning is compatible with copper and many copper alloys. In addition to degreasing and oxide removal, the process passivates the surface. It leaves a bright surface and extends the product’s shelf life (see lead photo).
It’s also effective for oxide removal on ferrous and nonferrous tube and pipe. This is a high-temperature process that allows simultaneous annealing. A cooling section is required.
Where surface activation is necessary, plasma prepares the surface for plating, spray coating, painting, extrusion coating, or taping. The process can use various gases, commonly nitrogen for copper alloys and hydrogen for ferrous materials. This is a low-temperature process, usually less than 300 degrees F.
Performed immediately before plating or coating, the plasma process replaces acid or alkaline cleaning, rinsing, and fluxing.
About the Authors
Igor Rogelj
13 Moorfield Road
Manchester, M20 2UZ
44-7810-810-656
Primoz Eiselt
Managing Director
13 Moorfield Road
Manchester, M20 2UZ
44-7810-810-656
About the Publication
Related Companies
subscribe now

The Tube and Pipe Journal became the first magazine dedicated to serving the metal tube and pipe industry in 1990. Today, it remains the only North American publication devoted to this industry, and it has become the most trusted source of information for tube and pipe professionals.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/30/2024
- Running Time:
- 53:00
Seth Feldman of Iowa-based Wertzbaugher Services joins The Fabricator Podcast to offer his take as a Gen Zer...
- Trending Articles
Zekelman Industries to invest $120 million in Arkansas expansion

3D laser tube cutting system available in 3, 4, or 5 kW

Corrosion-inhibiting coating can be peeled off after use

Brushless copper tubing cutter adjusts to ODs up to 2-1/8 in.

HGG Profiling Equipment names area sales manager

- Industry Events
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI
Precision Press Brake Certificate Course
- July 31 - August 1, 2024
- Elgin,