President
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Cleaning tube and pipe from the inside out
Vacuum cycling process agitates cleaning fluid to release lubricants, coolants, and debris
- By Donald Gray, Ph.D. and J.P. Schuttert
- October 31, 2019
- Article
- Tube and Pipe Fabrication
Regardless of how raw metal is turned into a tube or a pipe, the production process can leave quite a bit of residual material on the surface. Forming and welding on a mill, drawing on a draw bench, or using a pilger mill or extrusion press, followed by a cut-to-length process, can leave a tube or pipe surface grimy with lubricant and possibly littered with debris. Common contaminants that need to be removed from internal and external surfaces include drawing and cutting lubricants, both oil- and water-based; metal chips from cutting operations; and shop dust and debris.
Typical internal tube and pipe cleaning methods, whether aqueous or solvent, are the same as those used for cleaning external surfaces. These include flushing, swabbing, and ultrasonic cavitation. All of these methods are effective and have been used for decades.
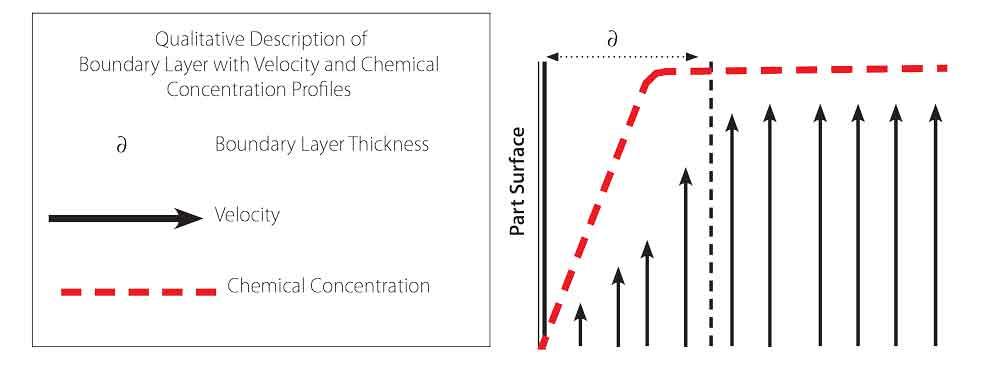
Of course, every process has limitations, and these cleaning methods are no exceptions to this rule. Flushing often requires manual manifolding and loses its effectiveness as the velocity of the flushing liquid decreases when the fluid approaches the tube’s surface, which is the boundary layer effect (see Figure 1). Swabbing can work well, but it is very labor intensive and is impractical on very small diameters, such as products for medical applications (hypodermic or lumen tubing). Ultrasonic energy is effective for cleaning exterior surfaces, but it doesn’t penetrate solid surfaces and has difficulty reaching the internal areas of tube, particularly when the product is bundled. Another drawback is that ultrasonic energy can cause surface damage. Sonic bubbles clean by cavitation, releasing large amounts of energy near the surface.
An alternative to these processes, vacuum cycling nucleation (VCN), causes bubbles to grow and collapse to move fluid. Fundamentally different from the ultrasonic process, it doesn’t risk damaging the metal’s surface.
Boiling at Room Temperature
VCN uses vapor bubbles to agitate and purge fluid from the inside of tube and pipe. An immersion process, it operates under vacuum and can be used with aqueous and solvent fluids.
It works on the same principle that causes vapor bubbles to form as water in a pan begins to boil. The first bubbles form at specific locations, especially in well-used pans. A close look at these locations usually reveals that these areas have rough spots or other surface defects. It is in these areas that the pan’s surface has more contact with a given fluid volume. Also, because these areas are shielded from natural convection cooling, bubbles form readily.
In boiling heat transfer, heat is transferred to the fluid to increase its temperature to the boiling point. When it reaches the boiling point, the temperature stops rising; adding more heat causes vapor to form, initially as vapor bubbles. When heat is added quickly, all the fluid on the surface becomes vapor, which is known as film boiling.
This is what happens any time a pan of water is brought to a boil; bubbles form initially at specific points on the pan’s surface, and later, when the water is roiling and churning, water evaporates quickly from the surface. Near the surface, it’s steam, which is invisible; as the steam cools through its contact with the surrounding air, it condenses into water vapor, which is easy to see as it forms above the pan.
Everyone knows that this happens at 212 degrees F (100 degrees C), but this isn’t complete. It happens at this temperature at standard atmospheric pressure, which is 14.7 pounds per square inch (PSI [1 bar]). In other words, on a day when the air pressure is 14.7 PSI at sea level, water boils at 212 degrees F at sea level; on the same day in that region, in a mountainous location 5,000 feet above sea level, the atmospheric pressure would be 12.2 PSI and water there would boil at 203 degrees F.
In other words, you can reduce the boiling point by reducing the atmospheric pressure.
Rather than raising the fluid temperature to the boiling point, the VCN process decreases the chamber pressure to the boiling point of the liquid at the ambient temperature. Just as in boiling heat transfer, once the pressure reaches the boiling point, the temperature and pressure remain constant. This pressure is known as the vapor pressure. When the internal surfaces of the tube or pipe have filled with vapor, the external surfaces supplement the vapor required to maintain the chamber at the vapor pressure.
Although boiling heat transfer illustrates the principles of VCN, the VCN process works in reverse of boiling.
A Selective Cleaning Process. Bubble nucleation is a selective process that targets specific areas for cleaning. Removing all of the air lowers the atmospheric pressure to 0 PSI, which is the vapor pressure, causing vapor to form on the surfaces. The growing vapor bubbles force fluid from the tube or pipe surface. When the vacuum stops, the chamber is returned to atmospheric pressure and purged; fresh fluid refills the tube for the next vacuum cycle. The vacuum/pressure cycle generally is set for 1 to 3 seconds and can be set to any number of cycles, adapting to the workpiece’s size and the contaminant.
The advantage of this process is it cleans the tube’s surface by starting at the contaminant site. As vapor grows, fluid is pushed along the tube’s surface and it accelerates, producing intensive agitation at the tube wall. The highest agitation is near the wall where the vapor grows. In essence, the process disrupts the boundary layer so that fluid adjacent to the surface remains high in chemical potential. Figure 2 shows two stages of the process using a surfactant at a 0.1 percent aqueous solution.
To form vapor, the bubbles need to form on a solid surface. This means that the cleaning process works from the surface to the fluid. Just as importantly, bubble nucleation starts with microscopic bubbles that coalesce on the surface to eventually form a stable bubble. Nucleation, therefore, prefers areas with high surface-area-to-liquid volume, such as tube and pipe IDs.
Vapor forms more easily inside the tube because of the tube’s concave curvature. Because bubbles form easily on the ID, vapor forms there first and rapidly enough to typically evacuate 70 to 80 percent of the liquid. The fluid near the surface at the peak of the vacuum stage is nearly 100 percent vapor, which simulates film boiling in boiling heat transfer.
The nucleation process works well on straight, bent, or coiled products in essentially any length or configuration.
Finding Hidden Savings. Aqueous systems using VCN can provide important cost savings. Because the process maintains higher chemical concentrations due to higher agitation near the tube surface (see Figure 1), it doesn’t require high concentrations of chemicals to promote chemical diffusion. The faster handling and cleaning attained also result in higher throughputs for a given machine, thus increasing the value of the equipment.
Finally, the VCN process, whether water- or solvent-based, enhances productivity by providing vacuum drying. This doesn’t require extra equipment; it’s simply part of the process.
One Process, Many Variations
Because of the enclosed design of the chamber and the temperature flexibility, a VCN system can be configured in many ways.

The vacuum cycling nucleation process is used to clean tubular components in sizes and applications as diverse as small-diameter medical products (left) and large-diameter waveguides used for radio signal transmission (right).
For solvent systems, in addition to VCN, other cleaning techniques such as vapor and spray can be used. In some unique applications, an ultrasonic system can be added to augment VCN. When solvents are used, the VCN process is supported by the vacuum-to-vacuum (or airless) process first patented in 1991. The process limits solvent emissions and usage to 97 percent or better. The process is recognized by both the Environmental Protection Agency and California’s South Coast Air Quality Management District for its effectiveness in limiting exposure and usage.
Solvent systems using VCN are cost-effective because every system is capable of vacuum distillation, which maximizes solvent recovery. This reduces solvent purchases and waste disposal. The process itself extends the service life of the solvent; the rate of solvent breakdown decreases as the operating temperature decreases.
If desired, these systems are adaptable to additional treatments such as passivation with acidic solutions or sterilization with hydrogen peroxide or other chemicals. The surface activity of the VCN process makes these treatments fast and cost-effective, and they can be combined in a single equipment design.
To date, VCN units in the field have treated tubing as small as 0.25 millimeter in diameter and tube having a diameter-to-wall-thickness ratio greater than 1,000 to 1. In lab studies, VCN has been effective in visual removal of internal contaminants in coiled tubes 1 meter long and 0.08 mm in diameter; in actual applications, it can clean through holes as small as 0.15 mm dia.
Information on chemistry consumption is available upon request.
Donald Gray, Ph.D., is president and J.P. Schuttert oversees sales for Vacuum Processing Systems, P.O. Box 822, East Greenwich, RI 02818, 401-397-8578, contact@vacuumprocessingsystems.com.
About the Authors
Donald Gray, Ph.D.
Vacuum Processing Systems
401-397-8578
J.P. Schuttert
Sales Director
Vacuum Processing Systems
401-397-8578
About the Publication
Related Companies
subscribe now

The Tube and Pipe Journal became the first magazine dedicated to serving the metal tube and pipe industry in 1990. Today, it remains the only North American publication devoted to this industry, and it has become the most trusted source of information for tube and pipe professionals.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
Zekelman Industries to invest $120 million in Arkansas expansion

3D laser tube cutting system available in 3, 4, or 5 kW

Corrosion-inhibiting coating can be peeled off after use

Brushless copper tubing cutter adjusts to ODs up to 2-1/8 in.

HGG Profiling Equipment names area sales manager

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI